UK Puberty Blockers Trial 2025: King's College London to Test Banned Drugs on 220 Children.
They banned the drugs for being unsafe. Now they're testing them on children to prove they're safe. Meanwhile, the pharma companies convicted of the largest fraud in history stand to make billions.
King’s College London will recruit 220 children under 16 for a clinical trial testing puberty blockers, starting January 2025. The UK government banned these same drugs in 2024 after the Cass Review found insufficient evidence of safety for under-18s. Dr Hilary Cass’s review concluded the drugs had a “very weak evidence base” and caused some children to experience “more negative than positive effects.”
They banned these drugs because there wasn’t enough evidence they were safe. Now their solution is to use kids as lab rats to find out if they’re safe.
The Trial Design
The Pathway trial will randomly assign 220 children into two groups: one receiving puberty blockers immediately, the other after a 12-month delay. The study will monitor bone density, brain development, and mental health over four years, with first results expected in 2029. Five to six children will be recruited monthly.
There’s no minimum age. Children as young as 11 could be enrolled. Prof Emily Simonoff, the study leader, admits they’re not expecting a “one size fits all finding” – translation: “We know this won’t work for everyone, but we’re going to do it to everyone anyway to find out who it doesn’t work for.”
Keira Bell – who took the Tavistock gender clinic to court in 2020 after receiving puberty blockers as a teenager – is threatening judicial review proceedings at the High Court to halt the trial. She’s been there, got the irreversible side effects. But apparently her testimony doesn’t count as “evidence.”
The Legal Paradox
Here’s the insanity: in the UK, it’s illegal to tattoo anyone under 18 – even a 17-year-old cannot legally consent to a tattoo under the Tattooing of Minors Act 1969. But an 11-year-old can apparently consent to puberty blockers that alter bone density, brain development, and fertility. A superficial mark on the skin requires you to be an adult. Fundamental, potentially irreversible changes to your developing body? That’s fine at 11.
There’s a reason children can’t vote, can’t marry, can’t sign contracts, can’t consent to sex, can’t get tattoos, can’t join the military. Every civilised legal system recognises that children require protection because they cannot fully comprehend long-term implications. Their brains are still developing. Their judgment is incomplete. Their prefrontal cortex won’t finish developing for another decade.
Medical experimentation on vulnerable populations requires not just consent but informed consent, and informed consent requires cognitive capacity an 11-year-old going through puberty, experiencing gender distress, under social and psychological pressure, does not have. The researchers can dress it up with “intensive screening” and “psychological support” and “strict criteria,” but you can’t screen your way around fundamental human development. You can’t counsel a child into having adult judgment.
What “long-term implications” means: bone density that may never recover, brain development altered in ways we don’t understand, fertility potentially compromised, sexual function possibly impaired, psychological effects we won’t know about for years. These aren’t reversible consequences. These are permanent alterations to a developing human being.
The Cass Review found that some children got worse on these drugs. Worse. Not “didn’t improve” – worse. The response is to give them to more children to find out exactly how much worse. Clinical data shows the majority of gender dysphoric youth naturally outgrow their condition without medical intervention. So we’re experimenting on children when we have good reason to believe that doing nothing would resolve the issue for most of them anyway.
Who Profits?
Dr Helen Webberley, stripped of her medical licence in July 2024, founded GenderGP. Her husband Mike prescribed puberty blockers to a nine-year-old child and cross-sex hormones to a teenager who later died by suicide. Despite losing their UK medical licences, GenderGP now operates from Singapore, providing puberty blockers to children as young as eight worldwide.
Webberley tells parents not to correct two-year-olds who think boys can have babies because we should “let children explore.” In debates with feminist Julie Bindel, she argued that women aren’t disproportionately rape victims, so rape crisis centres should include “everyone.” The UK banned her clinic, so she moved to America. In May 2024, the High Court stated there are “serious concerns as to the safety of patients accessing cross-hormone treatment from GenderGP.”
But Webberley’s operation is small-time compared to pharmaceutical giants who’ve turned gender dysphoria into a growth market.
The Multi-Billion Dollar Market
The US sex reassignment hormone therapy market was valued at $1.6 billion in 2022, projected to reach $2.23-2.57 billion by 2030-2033. The broader precocious puberty treatment market is projected to grow from $1.99 billion in 2025 to $3.99 billion by 2035.
One manufacturer, Endo Pharmaceuticals, discontinued its cheaper $4,800 puberty blocker (Vantas) while keeping the nearly identical version (Supprelin LA) that costs $43,000 – eight times more expensive. After discontinuing the cheaper option, revenues grew 79% year-over-year. A three-month supply of Lupron costs over $11,000.
Puberty blockers are just the entry point. Once on blockers, the typical pathway leads to cross-sex hormones, which are lifelong prescriptions. You’re creating customers for life, starting at age 11. Each transgender child reportedly represents over $1 million to the pharmaceutical industry.
Manufacturing Demand
These drugs – Lupron, Supprelin LA, Vantas – were FDA-approved to treat central precocious puberty, prostate cancer, and for chemical castration of sex offenders. They were NOT approved for gender dysphoria. But pharmaceutical companies found a new market: vulnerable children and desperate parents.
Texas Attorney General Ken Paxton launched investigations in 2021-2022 into AbbVie and Endo Pharmaceuticals under the Texas Deceptive Trade Practices Act for allegedly advertising puberty blockers for unapproved uses without disclosing risks. The drugs were FDA-approved only for precocious puberty and prostate cancer, not gender dysphoria.
The pharmaceutical playbook:
Normalize the ideology through advocacy groups (often pharma-funded)
Capture medical associations who issue “guidelines”
Train doctors to affirm rather than question
Market to parents through fear: “Would you rather have a living son or a dead daughter?”
Use activists as unpaid sales force
Fund “research” showing benefits while hiding harms
Get insurance to cover it
Profit
The relentless marketing creates children who question themselves – despite clinical data showing the majority naturally outgrow their condition without medical intervention. But if you can intercept them during that confusion and get them on the pharmaceutical pathway, you’ve got a customer for life.
Criminal History
In 2001, TAP Pharmaceutical Products paid $875 million – then the largest pharmaceutical settlement in history – to settle criminal and civil charges for illegally marketing Lupron. The kickbacks included trips to resorts, medical equipment, and “educational grants” of up to $65,000 to encourage doctors to prescribe Lupron over other drugs.
They took drugs for prostate cancer and sex offenders, repackaged them as “life-saving gender-affirming care,” and created a multi-billion dollar market out of confused children.
This King’s College London trial isn’t about science. It’s about market expansion. It’s about legitimising a revenue stream. It’s about creating the “evidence base” that allows the taps to be turned back on.
The Bottom Line
We have a multi-billion dollar market, growing at 4-7% annually, dominated by manufacturers already caught in the largest pharmaceutical fraud settlement in history, now positioning the same drugs for a new demographic of children.
The Cass Review was supposed to be the wake-up call. Instead, they’ve hit snooze and rolled over for another experiment. Different day, same kids, new consent forms.
They banned these drugs for private prescription and NHS use because they couldn’t prove they were safe. But clinical trials are apparently fine. So you can’t prescribe unproven drugs to kids unless you’re specifically testing unproven drugs on kids.
That’s not a loophole. That’s a moral chasm.
This isn’t civilised. This is barbarism dressed up in progressive language. This is ideology trampling over every ethical safeguard built up over decades of medical scandals and human rights abuses. This is adults using children’s bodies as pawns in commercial games, and calling it healthcare.
A civilised society protects children from exploitation. It protects them from adults who would use them for experiments. It protects them from irreversible decisions they’re not equipped to make.
This trial is the opposite of all that. It’s institutionalised child abuse with ethical approval and a profit margin.
The Almighty Gob is an independent journalist covering Bristol City Council and UK politics. Support independent journalism – subscribe for more accountability reporting.
Tags: #PubertyBlockers #CassReview #GenderDysphoria #KingsCollegeLondon #ChildSafeguarding #GenderGP #HelenWebberley #KeiraBell #UKHealthcare #MedicalEthics #PharmaceuticalProfits
Citations & Sources
UK Puberty Blockers Trial:
BBC News: “Details of new UK clinical trial to assess puberty-blocking drugs” (2024)
Cass Review:
https://cass.independent-review.uk/
King’s College London Pathway Trial announcement
Legal Framework:
Tattooing of Minors Act 1969 (UK Parliament)
Bell v Tavistock [2020] EWHC 3274 (Admin)
Helen Webberley / GenderGP:
General Medical Council tribunal findings (2022, 2024)
Medical Practitioners Tribunal Service records
High Court ruling re: GenderGP safety concerns (May 2024)
The Spectator: “Helen Webberley is terrifying” (November 2024)
Julie Bindel, UnHerd: “Trans activists still don’t understand the need for women’s refuges” (November 2024)
Pharmaceutical Market Data:
Grand View Research: “U.S. Sex Reassignment Hormone Therapy Market Report, 2030”
Vision Research Reports: “U.S. Sex Reassignment Hormone Therapy Market Size Set to Hit USD 2.57 Bn by 2033”
Future Market Insights: “Precocious Puberty Treatment Market Size & Forecast 2025-2035”
Global Market Insights: “U.S. Sex Reassignment Hormone Therapy Market Size Report, 2032”
Pharmaceutical Pricing & Marketing:
NPR/KHN: “Drug company drops its cheaper hormone-blocking drug, keeps pricier one” (November 2021)
Texas Attorney General press releases (December 2021, March 2022)
Office of the Texas Attorney General: Civil Investigative Demands to AbbVie and Endo Pharmaceuticals
Criminal Settlement:
U.S. Department of Justice: TAP Pharmaceutical Products settlement (2001)
The New York Times: “Drug Maker to Pay $875 Million for Fraud” (October 2001)
All claims in this article are supported by publicly available documents, court records, regulatory filings, and reputable news sources.


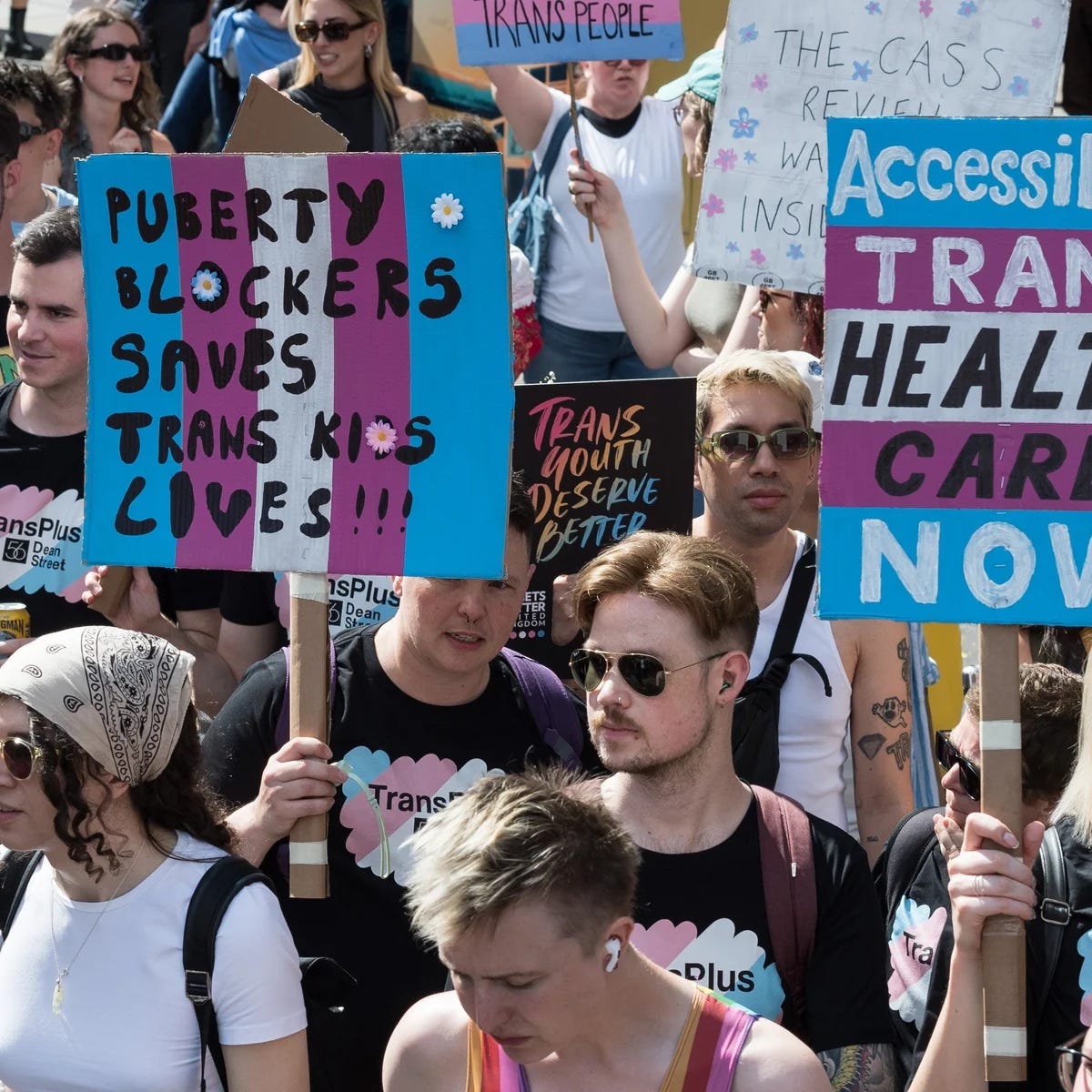
A friend committed suicide in 2017, ten years after her older sister did the same, and leaving three sons. The middle one was aged 12. In October he killed himself too, allegedly because nature had got him wrong and he couldn't deal with not being a girl/trying to be a girl but not being accepted - the medication presumably also played a role. It is devastatingly easy for people to blame nature for getting it wrong and others for their intolerance rather than looking at trauma and mental illness in a family and supporting a vulnerable child through this. Affirming a child's impossible beliefs about himself, and allowing him to latch onto the latest insane social contagion, rather than understanding he is attempting to block/numb the pain of abandonment by his mother, just keeps the whole family trapped in the awful cycle. It's heartbreaking.